Biological Time Series - Water Quality
3.4
Harmful Algal Blooms and the shellfish industry
Contributors
Steve Brett1
Claire Davies2
Ruth Eriksen2
Anthony J. Richardson3,4
1 Microalgal Services, Ormond, VIC, Australia
2 CSIRO Oceans and Atmosphere, Hobart, TAS, Australia
3 CSIRO Oceans and Atmosphere, Queensland Biosciences Precinct (QBP), St Lucia, QLD, Australia
4 Centre for Applications in Natural Resource Mathematics (CARM), School of Mathematics and Physics, The University of Queensland, St Lucia, QLD, Australia
Key Information
We found that HAB species of concern to the Australian shellfish industry are widely distributed in Southeast Australia. The dinoflagellate species most commonly above levels that trigger an industry response are Dinophysis acuminata and Dinophysis caudata. While Prorocentrum lima is often recorded above trigger levels, elevated toxins have not been detected in association with these events. Key HAB species have increased in abundance slightly over the past 10 years in Southern and Northern NSW, but are stable in Central NSW and Port Phillip Bay.
Keywords
Harmful Algal Blooms and the shellfish industry
Harmful Algal Blooms (HABs) occur when toxic or nuisance species of phytoplankton grow rapidly, leading to high abundances that can impact human health and marine life, and can often cause substantial economic losses to aquaculture, tourism, and hospitality industries. The most common problems with HABs in Australia are the accumulation of toxins in seafood that is then ingested by people, and fish kills from the toxins or from low dissolved oxygen in the water associated with the breakdown of phytoplankton blooms. Some phytoplankton species are problematic at low cell concentrations, i.e., even without a visible bloom. Because toxins can accumulate in the food chain, there is regular monitoring of marine species, particularly for shellfish harvest, to prevent impacts on human health. Monitoring usually includes a combination of phytoplankton community composition, environmental parameters, and analysis of toxin profiles in seafood products destined for human consumption.
Although HABs are natural, some might be exacerbated by global change, including eutrophication, climate change, and translocation (Wells et al., 2015, Hallegraeff, 2010).
Globally, the Intergovernmental Oceanographic Commission (Moestrup et al., 2009-2019) maintains a list of ~180 harmful phytoplankton species. Locally, public health, seafood safety and export agencies maintain lists of species deemed to be a risk in each particular region. These key species form the basis of monitoring programs. Here we analyse weekly phytoplankton data from a range of monitoring programs from the coast of mainland Southeast Australia to identify problem species, where they are found, their interannual trends and how often their levels exceed regulatory guidelines. Ultimately it is hoped that these data will help us understand the environmental triggers of blooms and toxin production.
Surface waters off the east coast of Australia have warmed by up to 2°C over the past 70 years, with greater warming in the south (e.g., Maria Island) than further north (e.g., Port Hacking) (Figure 1). This is a consequence of global warming and its influence on the intensification of the poleward-flowing East Australian Current (EAC) (Cai, Shi, Cowan, Bi, & Ribbe, 2005), which is distributing more warm-water to southern Australia (Ridgway, 2007). This increasing strength of the EAC has contributed to ocean warming off southeast Australia ~3–4 times the global average (Ridgway, 2007).
Here we compare the IMOS copepod data from three National Reference Stations in eastern Australia – Maria Island (42.6°S), Port Hacking (34.1°S) and Yongala (19.3°S). We chose these regions because there was historical data in each region to assess whether there is a signature of warming in the copepod community.
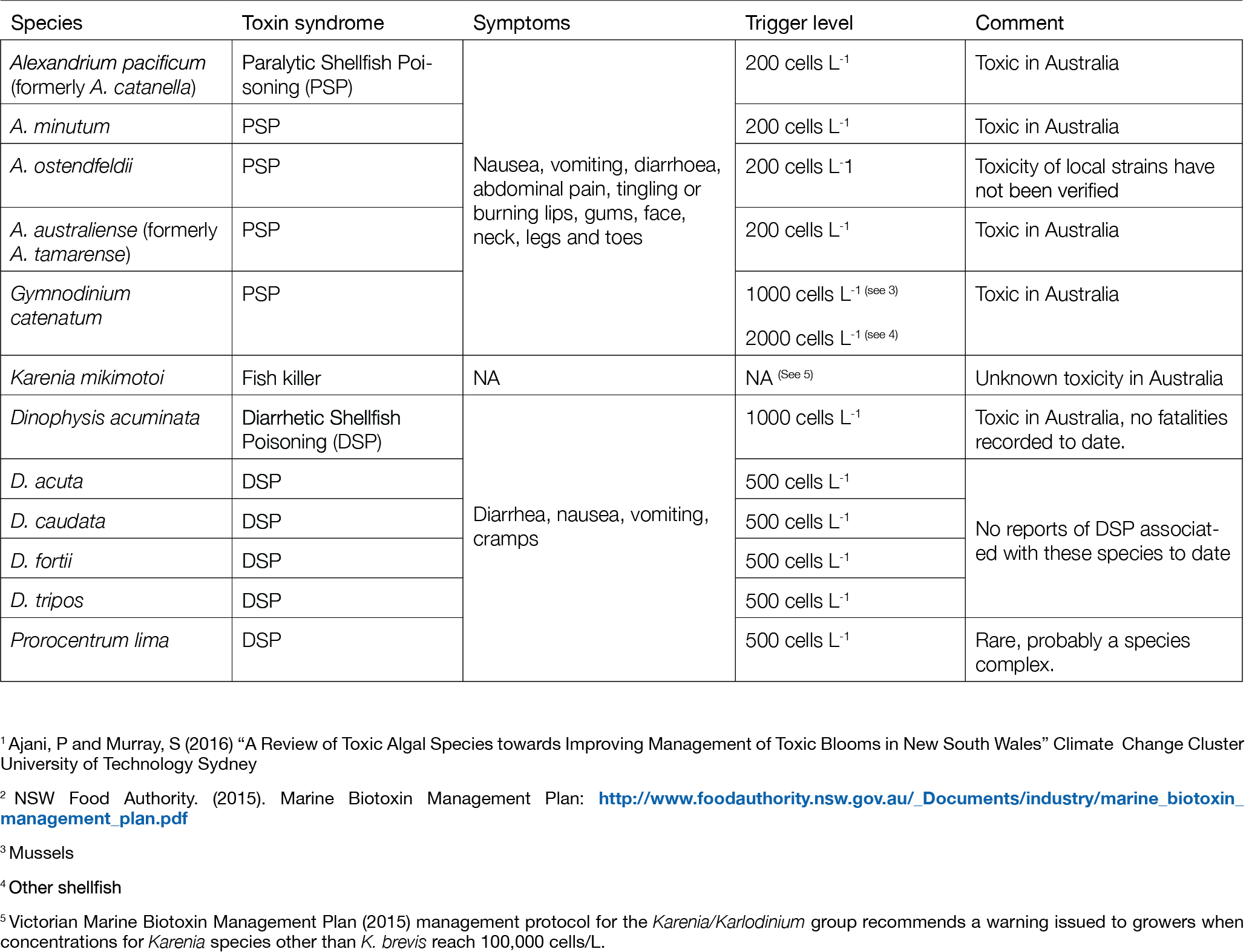
We analysed weekly data on HAB species collated in the Australian Phytoplankton Database (Davies et al., 2016) focusing on mainland Southeast Australia from 2003-2019. This includes data from shellfish industry monitoring programs and State and local government monitoring programs outside of aquaculture areas. This database compilation represents the largest publicly-available HAB dataset in Australia, and includes species that produce toxins accumulated by shellfish, as well as species that may cause other harmful or nuisance effects to fish or people. A subset of species monitored by the shellfish industry in Australia is shown in Table 1. It focuses on potentially harmful dinoflagellate species where robust species-level data are available through routine monitoring programs. Also shown are the relevant trigger levels or thresholds for each species. When the abundance of a HAB species is above its trigger level, further investigations including tissue testing of potentially affected shellfish is undertaken. Trigger levels were sourced from the NSW Phytoplankton Action Levels (NSW Food Authority, 2015) (Table 1).
For regional and seasonal analysis, we focused on 12 dinoflagellate species from five of the most common dinoflagellate genera. Southeast Australia was divided into four subregions that reflect the major areas of shellfish industry for which data are publicly available: Northern NSW, Central NSW, Southern NSW and Port Phillip Bay Victoria. We used Principal Components Analysis (PCA) to describe interannual and seasonal patterns in these regions for selected HAB dinoflagellate species. We used a hellinger transformation so that abundant species did not dominate. The 1st principal component scores were plotted so that most species had positive loadings (i.e., positive correlations with PC1).
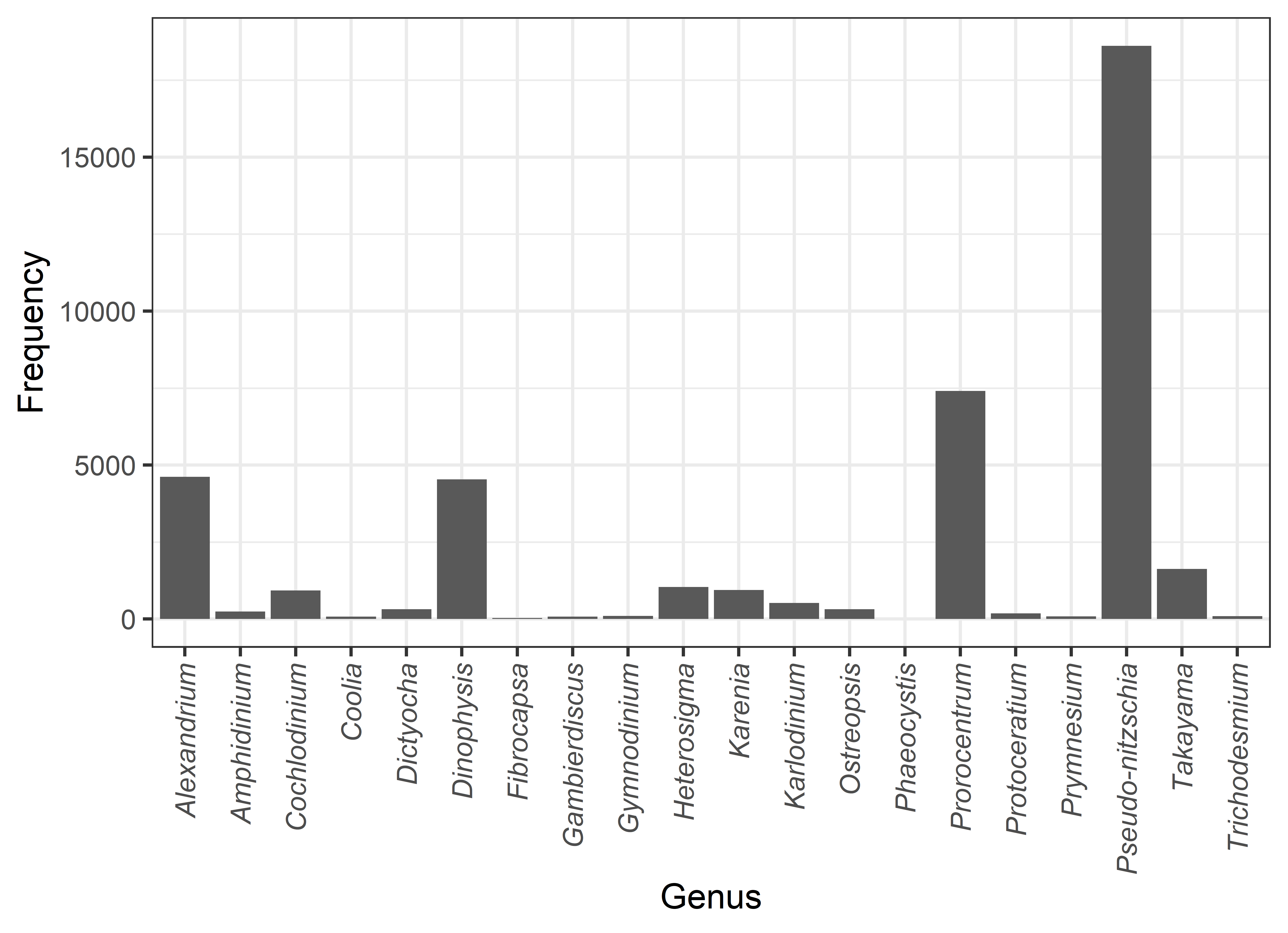
Over the 15 years of sampling in Southeast Australia, 20 HAB genera that produce toxins or cause problems for aquaculture or public amenity have been observed (Figure 1). The most common genus is the diatom Pseudo-nitzschia, although only some species in this group are toxic, and species-level detection is difficult with standard microscopy alone. Other common genera are the dinoflagellates Prorocentrum, Alexandrium and Dinophysis, which have many toxic species. Six genera are also associated with fish kills; viz. Amphidinium, Fibrocapsa, Heterosigma, Karenia, Karlodinium, and Takayama.
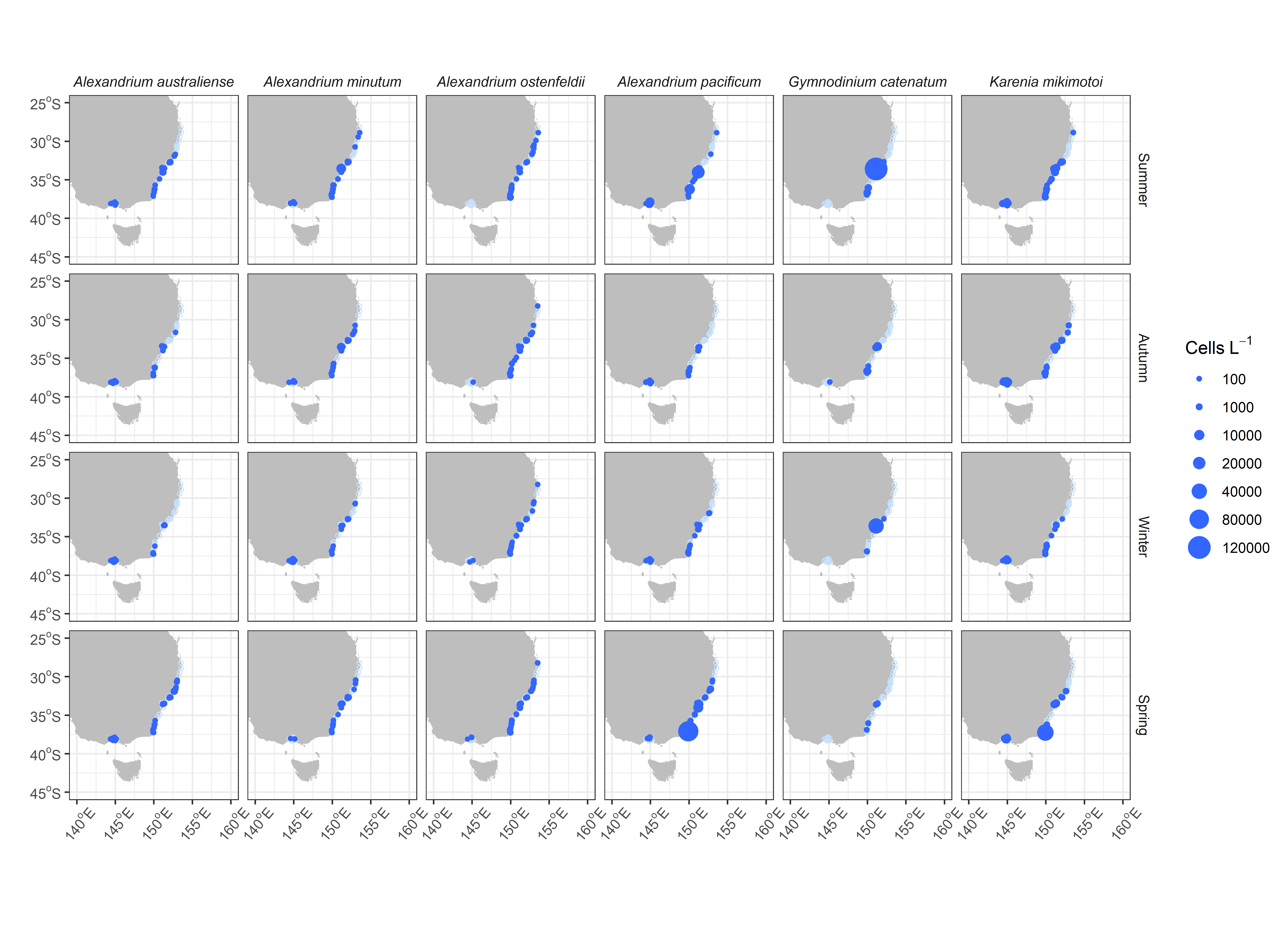
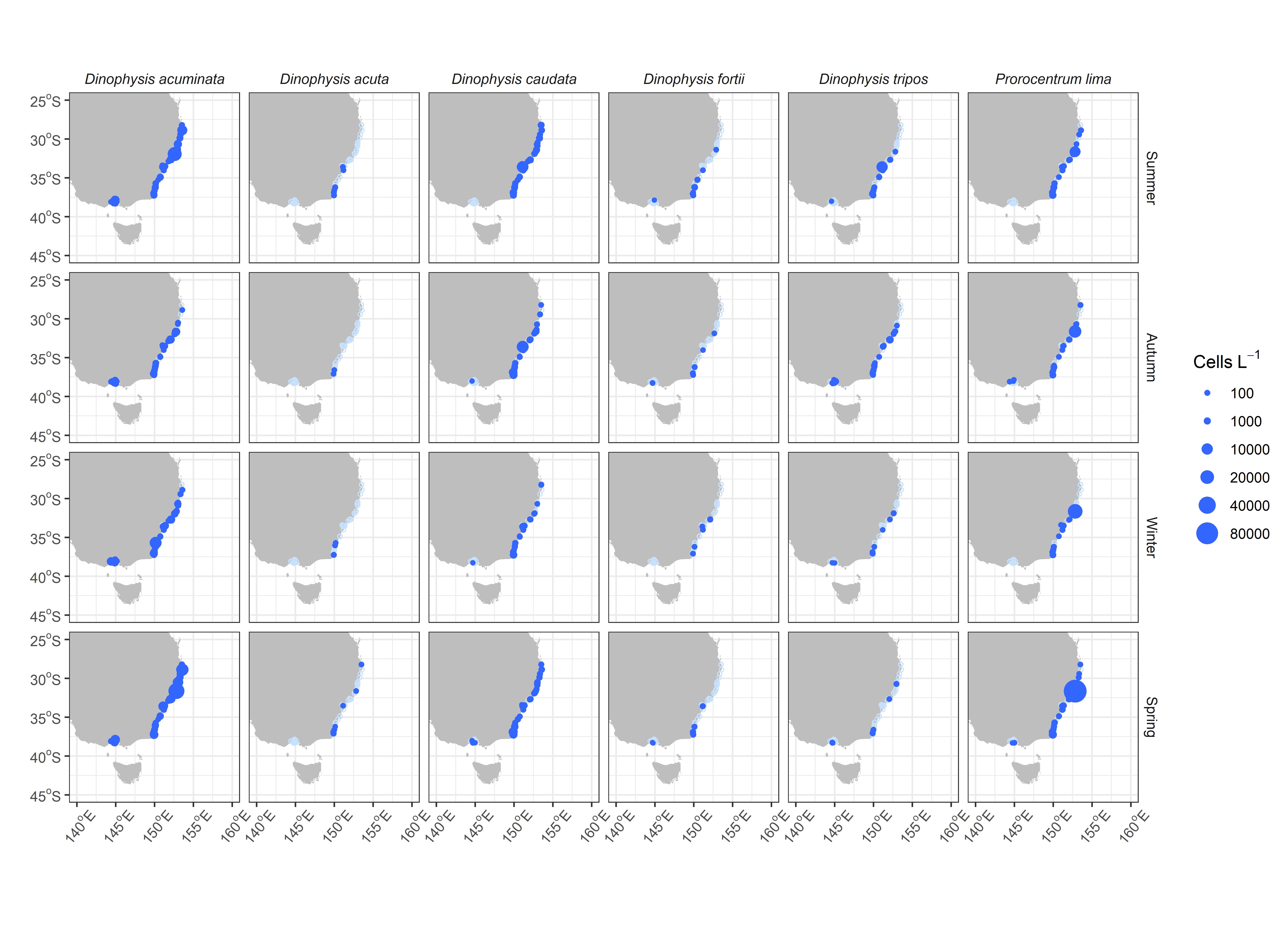
Our analysis of 12 dinoflagellates routinely discriminated to species level show there are some notable patterns in the seasonal distribution of these harmful species (Figure 2). In general, the dinoflagellate HAB species in the regions are not highly seasonal with most species found in all seasons, even in the colder waters of the south. Occasionally there is a large increase in one species e.g Gymnodinium catenatum in summer, Alexandrium pacificum and Prorocentrum lima in spring, however the increase is at a small number of sites rather than across the whole region. The most abundant species is Dinophysis acuminata, which is present throughout the year. Dinophysis caudata is found in modest numbers throughout all regions, in all seasons. Some species are relatively rare, including Dinophysis acuta6, Dinophysis fortii and Dinophysis tripos. Karenia mikimotoi, predominantly associated with fish-kills, has been observed in routine monitoring, in all regions.
Regionally, Central NSW appears to be a hotspot for HABs (Figure 3) with regular blooms of Alexandrium minutum, Alexandrium pacificum, Dinophysis caudata and Gymnodinium catenatum. In Northern NSW Gymnodinium catenatum has not been observed to date, and the four Alexandrium species of concern (A. australiense, A. minutum, A. ostenfeldii and A. pacificum) are also rarely observed there. Prorocentrum lima is observed routinely in this region at high concentrations, but is not commonly associated with toxin accumulation in shellfish. Other species, such as Alexandrium pacificum, Dinophysis acuminata and Karenia mikimotoi have been found in highest numbers in Southern NSW. In Port Phillip Bay, Victoria most species have been observed, but Gymnodinium catenatum and Prorocentrum lima are rare and Dinophysis acuta has not been recorded.
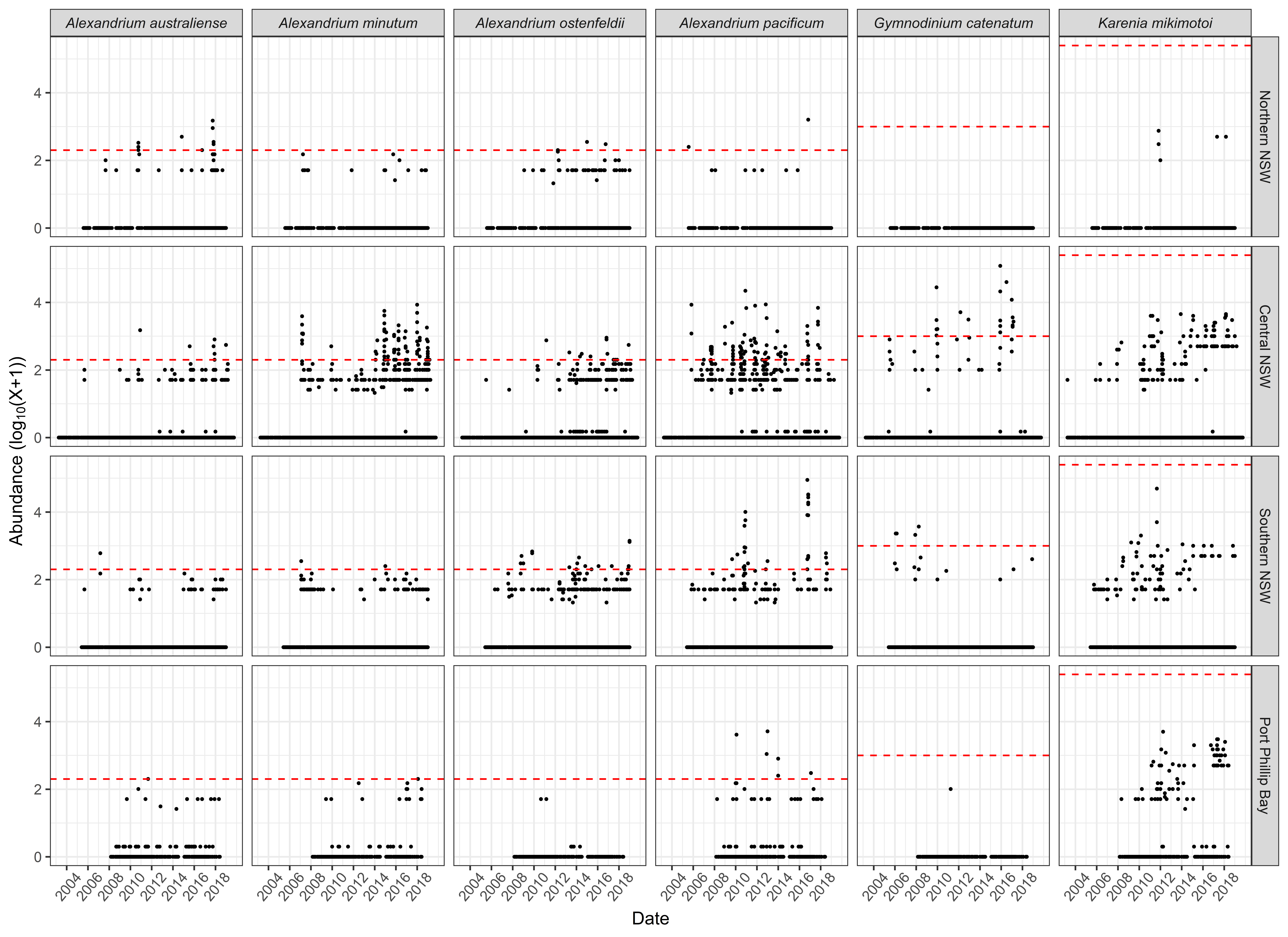
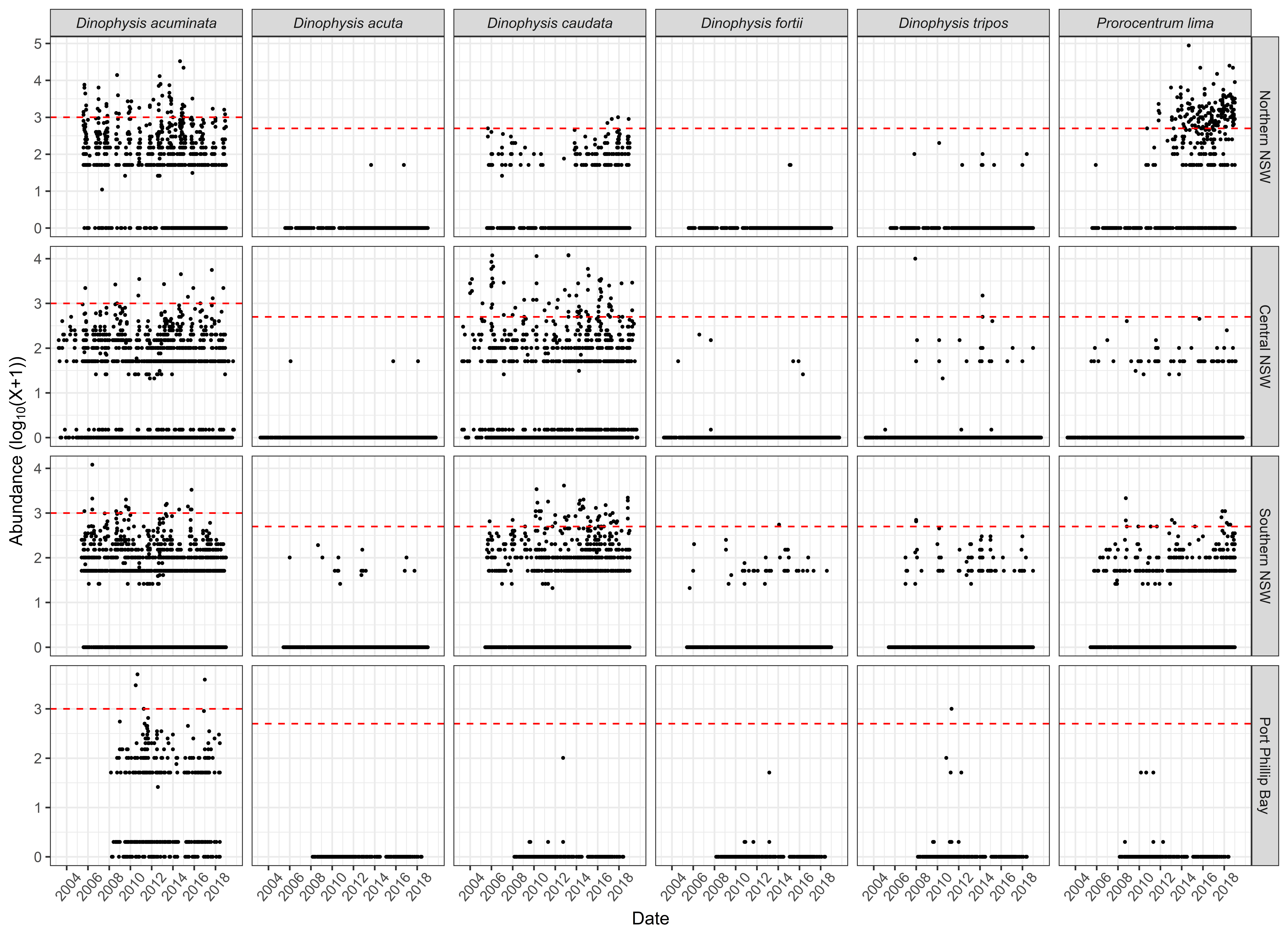
Monitoring of waters of Southeast Australia shows all the HAB species analysed here are most commonly found at background environmental levels, below aquaculture trigger levels (Figure 3). Two species, Dinophysis acuta and Karenia mikimotoi have never been above regulatory thresholds in any region. In Northern NSW, Prorocentrum lima is most commonly above regulatory thresholds (16.2%) and Dinophysis acuminata exceeds thresholds 6.0% of the time. In Central NSW three species exceeded their trigger levels about 3.5% of the time - Alexandrium minutum, Alexandrium pacificum and Dinophysis caudata. In Port Phillip Bay, HAB species are only rarely recorded above trigger levels.
Interestingly, there was a consistent pattern spatially in terms of the overall frequency of species recorded above trigger levels. It was greatest in the north and declined moving south: Northern NSW (2.0%), Central NSW (1.1%), Southern NSW (0.6%) and Port Phillip Bay (0.2%). There is also evidence of potential increases in the abundance of some HAB species in some regions. In Northern NSW, the abundance of Prorocentrum lima has increased, being regularly above trigger levels in recent years (although toxins are not elevated in these events). In Central NSW, Alexandrium minutum and Karenia mikimotoi have been observed more often at higher abundances, while Alexandrium australiense abundances have not. In Southern NSW there were also increases in Alexandrium pacificum, Dinophysis caudata and Karenia mikimotoi. Karenia mikimotoi in fact appears to have increased in abundance throughout mainland Southeast Australia.
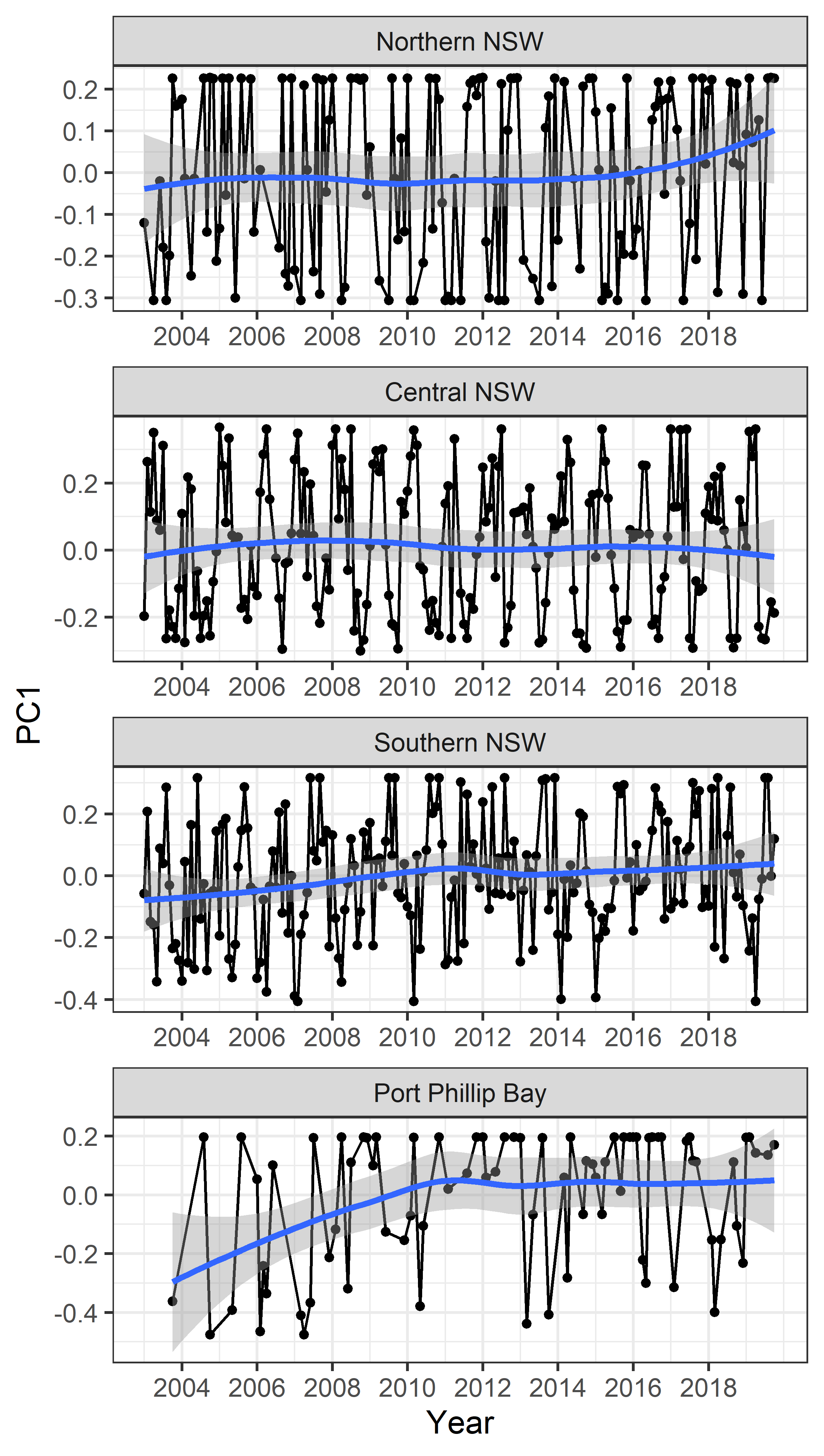
Using the first principal component (PC1) based on the abundance of the 12 dinoflagellate species to summarise long-term trends, we observed a slight change over time in most regions (Figure 4). In recent years there was an increase in the abundance of HAB species in Northern NSW, and a weak increase in Southern NSW. By contrast, Central NSW and Port Phillip Bay showed relatively stable trends in the abundance of HAB species over the past 10 years.
We found the key dinoflagellate HAB species analysed here that are problematic for the Australian shellfish industry are widely distributed around the coast of mainland Southeast Australia. The seasonal cycles of HAB species vary across Southeast Australia. The species most commonly above the levels that trigger a management response are Dinophysis acuminata, Dinophysis caudata and Prorocentrum lima, with each of those species having a different risk profile based on actual toxin production and accumulation in shellfish. Key HAB species have increased in abundance slightly over the past 10 years in Southern and Northern NSW, but show a weaker trend in Central NSW and are relatively stable in Port Phillip Bay.
Monitoring of harmful species is vital for protection of human health, and the reputation and value of our export markets. HABs can cause significant economic impact either through toxin production, non-toxin related impacts on the health of farmed and wild-caught species, or through loss of amenity. There is increased concern for changes in the frequency, intensity, and duration of HAB events in light of our changing marine environment. “Improved information on the linkages between HABs and climate will emerge only through the establishment and maintenance of long-term phytoplankton monitoring programmes adequately supported by environmental monitoring. At present, our understanding and ability to predict how climate may select for HABs are severely limited by the scarcity of long-term records” (Pitcher et al. 2018). Better understanding of environmental triggers for blooms and toxin production will improve monitoring and reduce risk around harvest closures and re-opening and is the focus of both research and management agencies.
Ajani, P and Murray, S (2016) “A Review of Toxic Algal Species towards Improving Management of Toxic Blooms in New South Wales” Climate Change Cluster University of Technology Sydney doi: 10.13140/RG.2.1.3816.2160
Davies, C. H., Coughlan, A., Hallegraeff, G., Ajani, P., Armbrecht, L., Atkins, N., . . . Richardson, A. J. (2016). A database of marine phytoplankton abundance, biomass and species composition in Australian waters. Scientific Data, 3, 160043. doi:10.1038/sdata.2016.43
Hallegraeff, G. M. (2010). Ocean climate change, phytoplankton community responses, and harmful algal blooms: A formidable predictive challenge. Journal of Phycology, 46(2), 220-235. doi: 10.1111/j.1529-8817.2010.00815.x
Moestrup, Ø., Akselmann, R., Fraga, S., Hoppenrath, M., Iwataki, M., Komárek, J., Zingone, A. E. (2009-2019). IOC-UNESCO Taxonomic Reference List of Harmful Micro Algae.
NSW Food Authority. (2015). Marine Biotoxin Management Plan: http://www.foodauthority.nsw.gov.au/_Documents/industry/marine_biotoxin_management_plan. pdf
VICTORIAN MARINE BIOTOXIN MANAGEMENT PLAN (2015) https://vfa.vic.gov.au/aquaculture/publications/shellfish-quality-asurance/marine-biotoxin-management-plan
Wells, M. L., Trainer, V. L., Smayda, T. J., Karlson, B. S. O., Trick, C. G., Kudela, R. M., . . . Cochlan, W. P. (2015). Harmful algal blooms and climate change: Learning from the past and present to forecast the future. Harmful Algae, 49, 68-93. doi:10.1016/j.hal.2015.07.009
Figure 1
The frequency of occurrence of harmful algal bloom genera around the coast of mainland Southeast Australia. Not all genera are associated with toxin production, other effects include gill irritation in fish, visual impacts and skin or respiratory irritation.
Figure 2
Spatial distribution of key HAB dinoflagellate species by season, across mainland Southeast Australia. Empty circles (light blue) represent samples collected and analysed but no cells were detected for that species.
Figure 2
Spatial distribution of key HAB dinoflagellate species by season, across mainland Southeast Australia. Empty circles (light blue) represent samples collected and analysed but no cells were detected for that species.
Figure 3
Trends in the abundance of selected dinoflagellate HAB species in Southeast Australia, in relation to their trigger levels for warning growers and further testing of shellfish in aquaculture areas. Note the dots representing zero abundance for each HAB species, and that abundances are log10 transformed.
Figure 3
Trends in the abundance of selected dinoflagellate HAB species in Southeast Australia, in relation to their trigger levels for warning growers and further testing of shellfish in aquaculture areas. Note the dots representing zero abundance for each HAB species, and that abundances are log10 transformed.
Figure 4
The first principal component (PC1) representing abundance of the dinoflagellate HAB species through time, in the four subregions in Southeast Australia.
Table 1
Summary of dinoflagellate species included in this analysis, and their associated shellfish toxin syndrome or other major impacts (see 1 for more details). Trigger levels listed for shellfish are based on the NSW Marine Biotoxin Management Plan (2015)2. Discrimination of the species listed is based on stable morphological characteristics that can be routinely observed by microscopy, by suitably trained personnel.
Download this Time Series Report
Citing this report:
Brett S, Davies C, Eriksen R, Richardson A.J. (2020). Harmful algal blooms in the shellfish industry. In Richardson A.J, Eriksen R, Moltmann T, Hodgson-Johnston I, Wallis J.R. (Eds). State and Trends of Australia’s Ocean Report. doi: 10.26198/5e16ac8149e83
doi: 10.26198/5e16ac8149e83
Citing the Report
Richardson A.J, Eriksen R, Moltmann T, Hodgson-Johnston I, Wallis J.R. (2020). State and Trends of Australia’s Ocean Report, Integrated Marine Observing System (IMOS).

The State and Trends of Australia's Ocean Report was supported by IMOS. IMOS gratefully acknowledges the additional support provided by the Commonwealth Scientific and Industrial Research Organisation (CSIRO).
The State and Trends of Australia's Ocean website is maintained by IMOS.

Australia’s Integrated Marine Observing System (IMOS) is enabled by the National Collaborative Research Infrastructure Strategy (NCRIS). It is operated by a consortium of institutions as an unincorporated joint venture, with the University of Tasmania as Lead Agent.
Disclaimer:
You accept all risks and responsibility for losses, damages, costs and other consequences resulting directly or indirectly from using this site and any information or material available from it. While the Integrated Marine Observing System (IMOS) has taken reasonable steps to ensure that the information on this website and related publication is correct, it provides no warranty or guarantee that information provided by the authors is accurate, complete or up-to-date. IMOS does not accept any responsibility or liability for any actions taken as a result of, or in reliance on, information on its website or publication. Users should check with the originating authors to confirm the accuracy of the information before taking any action in reliance on that information.
If you believe any information on this website or in the related publication is inaccurate, out of date or misleading, please bring it to our attention by contacting the authors directly or emailing us at IMOS@imos.org.au
Images and Information:
All information on this website remains the property of those who authored it. All images on this website are licensed through Adobe Stock, Shutterstock, or have permission from the original owner.