Biological Time Series - Water Quality
3.2
Spatial and seasonal trends in Trichodesmium
Contributors
Claire Davies1
Ruth Eriksen1
Anthony J. Richardson2,3
1 CSIRO Oceans and Atmosphere, Hobart, TAS, Australia
2 CSIRO Oceans and Atmosphere, Queensland Biosciences Precinct (QBP), St Lucia, QLD, Australia
3 Centre for Applications in Natural Resource Mathematics (CARM), School of Mathematics and Physics, The University of Queensland, St Lucia, QLD, Australia
Key Information
Trichodesmium is an important nitrogen fixer especially in oligotrophic areas of the oceans and a major component of primary production. Monitoring the changes in abundances and seasonal variations in Trichodesmium around Australia help us to understand which environmental variables are driving abundance and distribution. It appears that sea surface temperature and phosphate availability are the major drivers for increased abundances in the GBR. The increased abundances we are seeing over time at the Yongala National Reference Station may be a response to climate change and will have implications for nutrient cycling in the region.
Keywords
sea surface temperature, nutrient cycling, climate change
Spatial and seasonal trends in Trichodesmium
Trichodesmium is a large filamentous marine cyanobacterium (Figure 1), notable for its ability to fix atmospheric nitrogen. It forms extensive, high biomass surface blooms visible from space. These blooms are known in Australia as “sea sawdust” and were noted by Captain James Cook sailing down the Great Barrier Reef in 1770. Trichodesmium blooms can be a major contributor to primary production in tropical systems. By fixing atmospheric nitrogen, Trichodesmium introduces “new” nitrogen into the low-nutrient waters (Blondeau-Patissier, Brando, Lønborg, Leahy, & Dekker, 2018), supplementing the limited regenerated nitrogen. Trichodesmium is important in the global nitrogen cycle and is the only genus resolved in the eReefs biogeochemical model of the Great Barrier Reef (Skerratt et al., 2019).
When present in high densities, Trichodesmium can provide food and habitat for a diverse range of organisms including zooplankton (O'Neil, 1998) (Figure 1). Trichodesmium produces potent neurotoxins, causing respiratory distress and contact dermatitis in humans (Schock, Huncik, Beauchesne, Villareal, & Moeller, 2011). Trichodesmium thus has potential for significant environmental and economic impacts. Large blooms or surface expressions of Trichodesmium typically indicate cells and filaments no longer actively growing, and have ceased their characteristic vertical migration in the water column they use to search for optimal nutrient conditions (Villareal & Carpenter, 1990).
Whilst strongly associated with the tropics due to its preference for warm waters (the mean Species Temperature Index is 26.1oC from IMOS records), the genus is widely observed in the IMOS Continuous Plankton Recorder and National Reference Station samples (typically at low concentrations but occasionally in blooms) around Australia. A variety of morphological forms are observed, but true species diversity is poorly understood, and is currently the focus of genetic studies.
Time-series observations of Trichodesmium around Australia were compiled from counts at the IMOS National Reference Stations (Eriksen et al. (2019) and the Australian Continuous Plankton Recorder survey (see Overall Methods Figure X). At the IMOS National Reference Stations, we used counts from zooplankton net samples rather than abundance estimates from phytoplankton bottles as is more typical. This was because zooplankton net samples (volume of water sampled = 10s of m3 water sampled) had far fewer absences of Trichodesmium than phytoplankton bottle samples (several litres) because of the larger volume sampled. We used additional observations of Trichodesmium from the Australian Phytoplankton Database available on the AODN (Davies et al., 2016). All data were sourced from the AODN (https://portal.aodn.org.au/; see the datasets “IMOS National Reference Station (NRS) - Zooplankton Abundance”, “IMOS - AusCPR: Phytoplankton Abundance”, and “The Australian Phytoplankton Database (1844 - ongoing) - abundance and biovolume”).
As Trichodesmium is most abundant at the Yongala National Reference Station, we explored its environmental drivers there. We used a linear model to investigate how abundance is related to a suite of environmental drivers including sea surface temperature, phosphate concentration, mixed layer depth, and month at this site. We used a harmonic term – a mix of sine and cosine waves – to model the seasonal cycle. SST data were sourced from GHRSST (http://rs-data1-mel.csiro.au/thredds/catalog.sstL3Syts.html?dataset=l3s_sst_day_1dts) and Chl-a data from MODIS ( http://rs-data1-mel.csiro.au/thredds/dodsC/imos-srs/oc/aqua/). Phosphate concentration and mixed layer depth were from concurrent measurements at Yongala (https://portal.aodn.org.au/; see the dataset “IMOS National Reference Station (NRS) - Salinity, Carbon, Alkalinity, Oxygen and Nutrients (Silicate, Ammonium, Nitrite/Nitrate, Phosphate”). Predictors were retained in the model based on AIC (Akaike’s Information Criterion).
To investigate the spatial distribution of Trichodesmium around Australia and how it varies seasonally, we developed a species distribution model using a generalised linear model using a suite of environmental predictors, including sea surface temperature, phosphate concentration, water column depth (bathymetry) and month. We used a negative binomial error structure, which can better model count data with a preponderance of zeros than a more typical Poisson distribution.
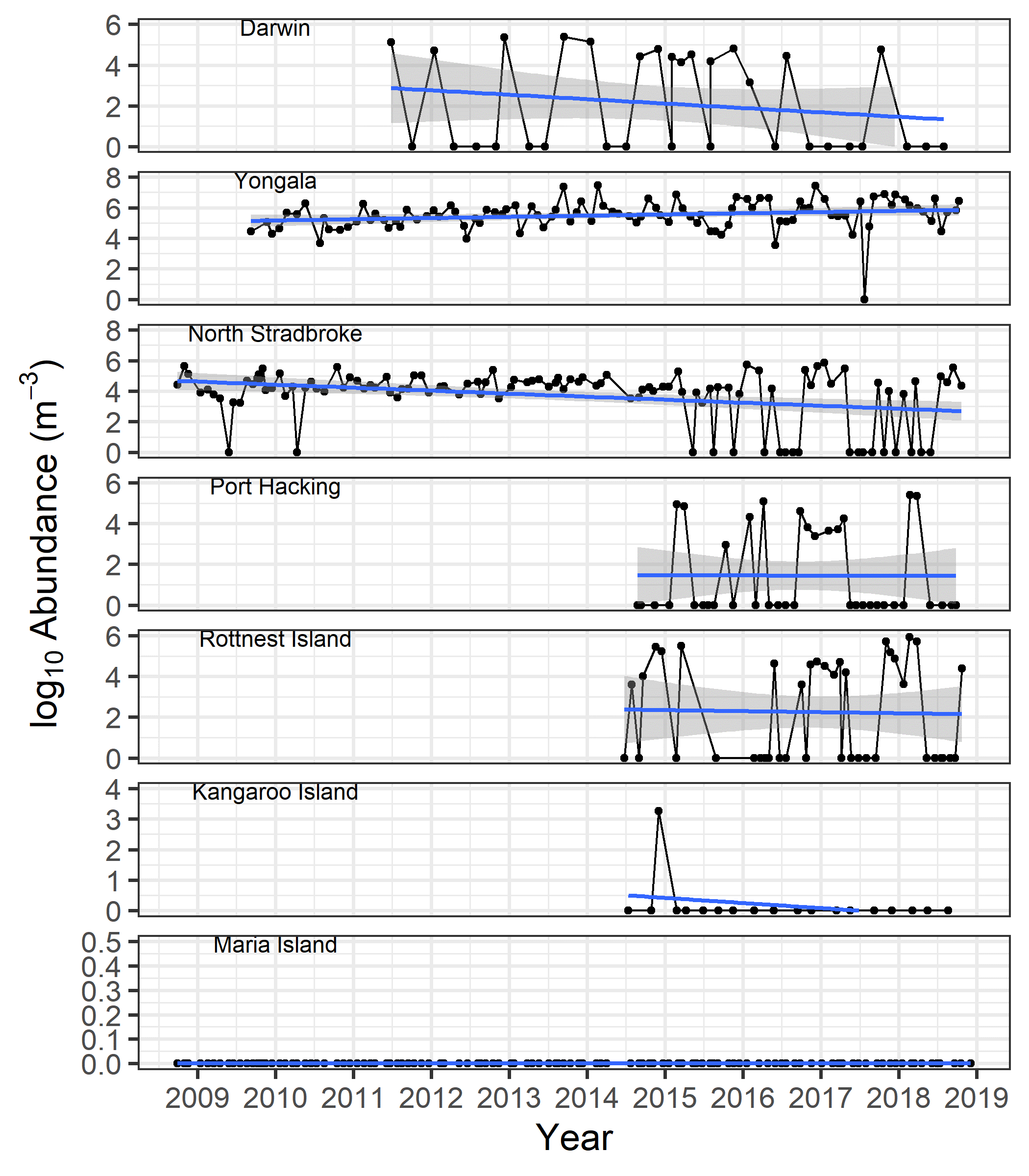
Trichodesmium has been observed at almost all locations sampled around the Australian coastline, although it is rare outside tropical waters. Its presence in more southerly and seasonally cooler waters is often the result of prevailing winds and currents. In tropical waters of Yongala, Trichodesmium has been observed in all but one of the monthly net samples collected since 2009 (n = 120), and an increase in abundance has been observed over the monitoring period (Figure 2). Abundances at Darwin, Rottnest Island, North Stradbroke Island and Port Hacking are generally lower, and vary more seasonally than at Yongala (Figure 2). Overall, abundances have declined significantly at North Stradbroke Island over the past decade. Trichodesmium is rarely observed in southern Australia, with one occurrence at Kangaroo Island and it has never been seen at Maria Island.
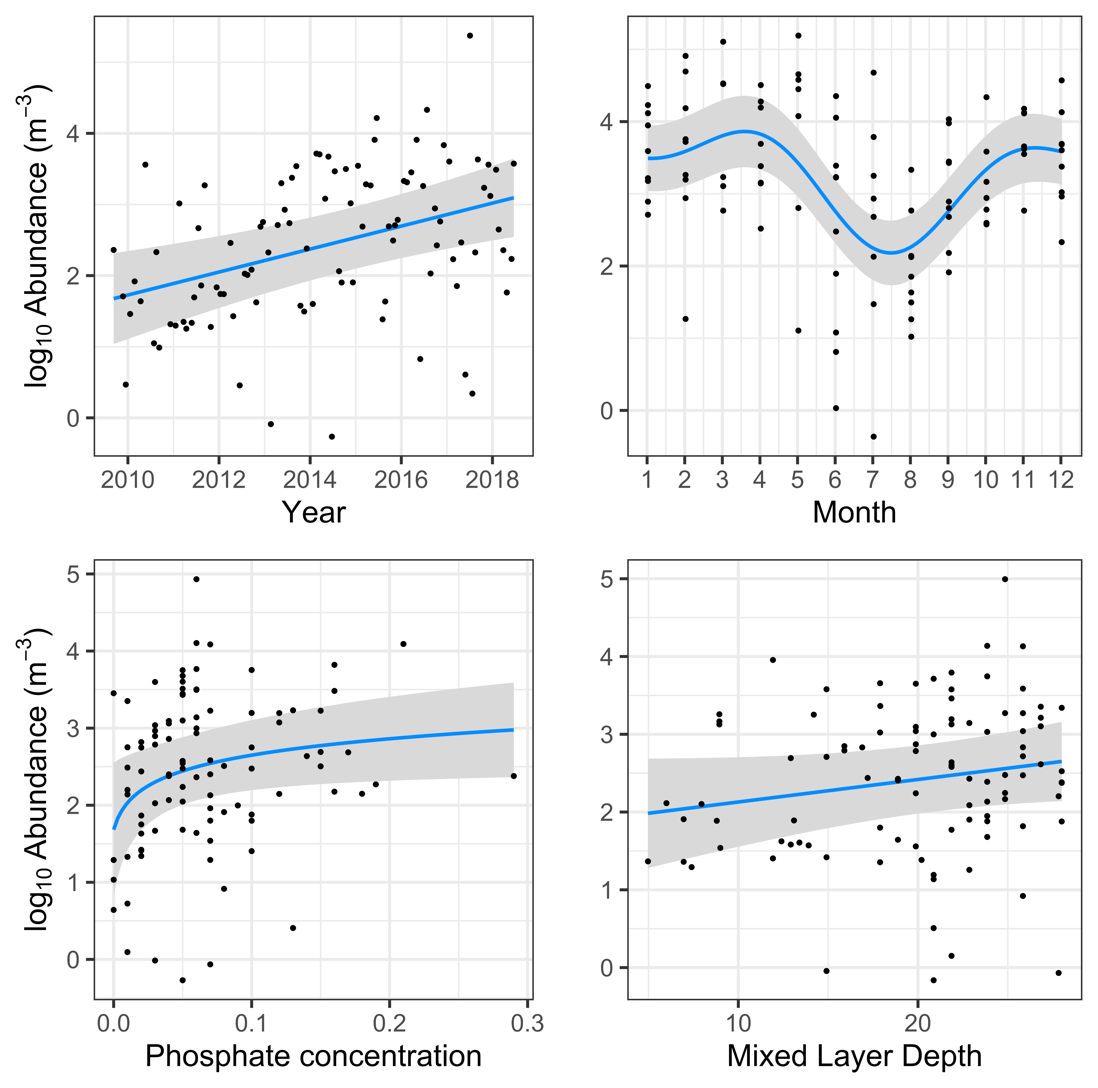
At Yongala, the strongest predictor of Trichodesmium abundance was the seasonal cycle, with abundance increasing in spring, dipping slightly in summer, before increasing again in autumn and declining in winter (Figure 3). The next most important predictor was Year, exhibiting a strong increasing trend in Trichodesmium abundance. We also found that more phosphate and deeper mixed layers corresponded to higher Trichodesmium abundances. SST, chlorophyll-a and iron concentration were not significant.
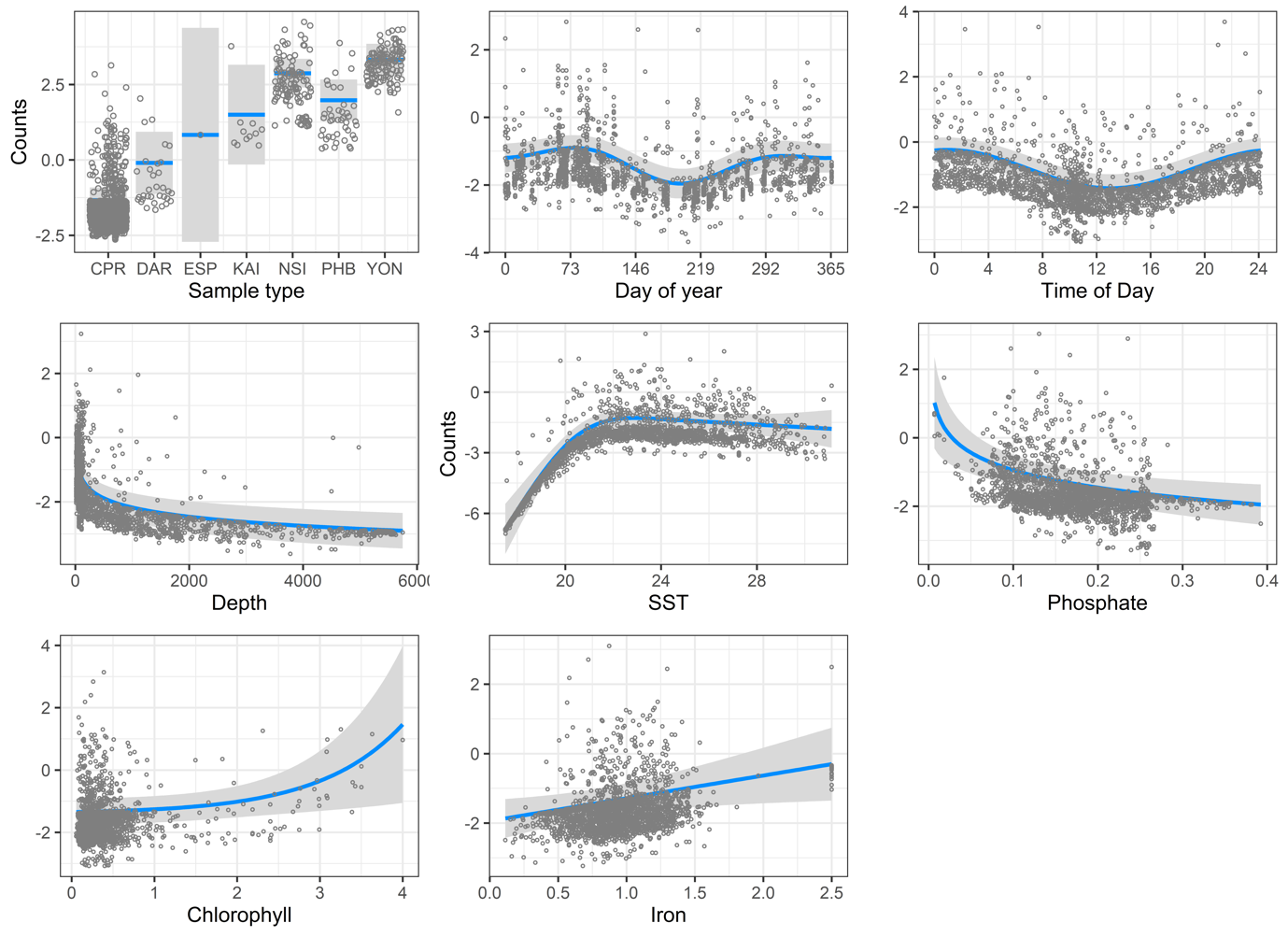
The generalised linear model of Trichodesmium around Australia showed that the more tropical National Reference Stations (Yongala and North Stradbroke Island) had higher Trichodesmium abundance compared with other areas (Figure 4). The decline in abundance from northern to southern areas is evident. There was also a marked seasonal cycle, very similar to that observed at Yongala. There were high abundances in spring and autumn, with a slight dip in summer, and a large dip in winter. There is evidence for vertical migration, with higher Trichodesmium abundances at night (note that we could not test for this at Yongala because all samples were collected during the day). In terms of bathymetry, the Trichodesmium abundance declines strongly offshore. Trichodesmium is rare in water <20oC, peaks in water of ~23oC, then the abundance declines gently to 31oC. This is similar to other studies that have found a preferred temperature niche of 24-29oC (Bergman, Sandh, Lin, Larsson, & Carpenter, 2013).
Counterintuitively, Trichodesmium was inversely related to phosphate concentration, in contrast to the model from Yongala alone. Trichodesmium abundance increases with iron concentration, similar to the model for Yongala. Trichodesmium abundance increased with chlorophyll-a concentration, whereas chlorophyll-a was found not to be important at Yongala.
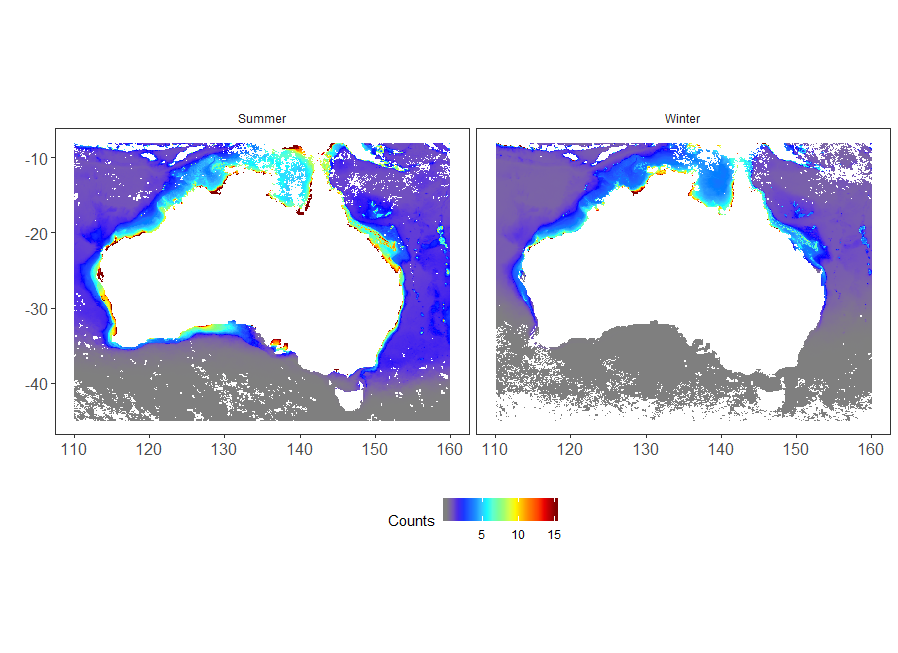
The map of the distribution of Trichodesmium based on the generalised linear model shows a tropical inshore distribution (Figure 5). Temperature was the most significant driver, and bathymetry was also important in determining Trichodesmium abundance (Figure 4). The model provides an integrated picture of the seasonal distribution of Trichodesmium, as well as expected abundance in areas that have not been sampled, or only sampled infrequently such as the North West Coast (Figure 5). It is clear that Trichodesmium extends further south during summer.
We found a significant increase in Trichodesmium abundance at Yongala. This increase will enhance the capture of new nitrogen into the oligotrophic waters of the region and will undoubtedly have implications for nutrient cycling. The increase is consistent with hypothesised impacts of climate change on phytoplankton communities in nutrient-poor regions. Because of enhanced stratification and the subsequent decline in surface nutrient conditions with climate change, nitrogen fixers such as Trichodesmium are expected to benefit (Beardall & Stojkovic, 2006).
Although surface expressions of Trichodesmium may extend for many hundreds of kilometres (Blondeau-Patissier et al., 2018) it is currently unknown whether the increase in Trichodesmium at Yongala is indicative of a broader phenomenon across the Great Barrier Reef. We also noted an overall decline in Trichodesmium abundance at North Stradbroke Island, although causes of this change are unclear. Although changes in Trichodesmium abundance can have large effects on nutrient cycling, the degree to which Trichodesmium is used directly by higher trophic levels is also a subject of debate. There is a small but obligate community that lives on it, which includes the harpacticoid copepod, Macrosetella gracilis (Figure 1f). This species uses the filaments as a food source and as a protective substrate when young (O’Neil 1998).
Our observations confirm that Trichodesmium is a tropical species, with high abundances at warmer temperatures and in warmer months (although slightly lower in the middle of summer). We see the distribution of Trichodesmium extending further south during summer and receding north during winter. The southerly extent of Trichodesmium could be a good indicator of climate change. We will be monitoring whether it is making more frequent and deeper incursions into southerly areas. This is similar to the red tide species Noctiluca, which we have seen increase its range further south.
Beardall, J., & Stojkovic, S. (2006). Microalgae under global environmental change: Implications for growth and productivity, populations and trophic flow. ScienceAsia, 32. doi:10.2306/scienceasia1513-1874.2006.32(s1).001
Bergman, B., Sandh, G., Lin, S., Larsson, J., & Carpenter, E. J. (2013). Trichodesmium– a widespread marine cyanobacterium with unusual nitrogen fixation properties. FEMS Microbiology Reviews, 37(3), 286-302. doi:10.1111/j.1574-6976.2012.00352.x
Blondeau-Patissier, D., Brando, V. E., Lønborg, C., Leahy, S. M., & Dekker, A. G. (2018). Phenology of Trichodesmium spp. blooms in the Great Barrier Reef lagoon, Australia, from the ESA-MERIS 10-year mission. PLoS ONE, 13(12), e0208010. doi:10.1371/journal.pone.0208010
Davies, C. H., Coughlan, A., Hallegraeff, G., Ajani, P., Armbrecht, L., Atkins, N., . . . Richardson, A. J. (2016). A database of marine phytoplankton abundance, biomass and species composition in Australian waters. Scientific Data, 3, 160043. doi:10.1038/sdata.2016.43
Eriksen, R. S., Davies, C. H., Bonham, P., Coman, F. E., Edgar, S., McEnnulty, F., . . . Richardson, A. J. (2019). Australia’s long-term plankton observations: the Integrated Marine Observing System National Reference Station network. Frontiers in Marine Science.
O'Neil, J. M. (1998). The colonial cyanobacterium Trichodesmium as a physical and nutritional substrate for the harpacticoid copepod Macrosetella gracilis. Journal of Plankton Research, 20(1), 43-59. doi:10.1093/plankt/20.1.43
Schock, T., Huncik, K., Beauchesne, K., Villareal, T., & Moeller, P. (2011). Identification of Trichotoxon, a novel chlorinated compound associated ith the bloom forming cyanobacterium Trichodesmium thiebautii. Environmental Science & Technology, 45, 7503-7509. doi:dx.doi.org/10.1021/es201034r
Skerratt, J. H., Mongin, M., Wild-Allen, K. A., Baird, M. E., Robson, B. J., Schaffelke, B., . . . Steven, A. D. L. (2019). Plankton and nutrient dynamics on the Great Barrier Reef: Skill assessment and analysis of the eReefs biogeochemical model. Journal of Marine Systems, 192, 51-74.
Villareal, T., & Carpenter, E. (1990). Diel buoyancy regulation in the marine diazotrophic cyanobacterium Trichodesmium thiebautii. Limnology and Oceanography, 35(8), 1832-1837.
Figure 1
Trichodesmium morphology from the east coast of Australia. Straight filamentous forms from Yongala viewed under a) a light microscope (scale bar 100 μm) and b) a scanning electron microscope (scale bar 10 μm).c) A “Tuft” form from Yongala (scale bar 100 μm) and d) a “puff” form from Port Hacking (scale bar 100 μm). e) A composite collection of various forms of filamentous cyanobacteria from Port Hacking (scale bar 500 μm). Images a) Ruth Eriksen CSIRO, b) Gustaaf Hallegraeff IMAS, c-e) Julian Uribe Palomino CSIRO (see Robson et al. in prep.). f) The marine copepod Macrosetella gracilis, which uses Trichodesmium as a food source and physical substrate for all aspects of its life-cycle (image: A. Slotwinki CSIRO).
Figure 2
Trichodesmium abundance at the IMOS National Reference Stations, based on counts of monthly zooplankton net samples.
Figure 3
Effect plots for the linear model at the Yongala NRS, showing the influence of key parameters on Trichodesmium abundance.
Figure 4
Negative binomial model output for distribution and abundance of Trichodesmium around Australia.
Figure 5
Seasonal distribution maps of Trichodesmium abundance, based on a negative binomial error structure with a suite of environmental predictors.
Download this Time Series Report
Citing this report:
Davies C, Eriksen R, Richardson A.J. (2020) Spatial and Seasonal trends in Trichodesmium. In Richardson A.J, Eriksen R, Moltmann T, Hodgson-Johnston I, Wallis J.R. (Eds). State and Trends of Australia’s Ocean Report. doi: 10.26198/5e16abb949e81
doi: 10.26198/5e16abb949e81
Citing the Report
Richardson A.J, Eriksen R, Moltmann T, Hodgson-Johnston I, Wallis J.R. (2020). State and Trends of Australia’s Ocean Report, Integrated Marine Observing System (IMOS).

The State and Trends of Australia's Ocean Report was supported by IMOS. IMOS gratefully acknowledges the additional support provided by the Commonwealth Scientific and Industrial Research Organisation (CSIRO).
The State and Trends of Australia's Ocean website is maintained by IMOS.

Australia’s Integrated Marine Observing System (IMOS) is enabled by the National Collaborative Research Infrastructure Strategy (NCRIS). It is operated by a consortium of institutions as an unincorporated joint venture, with the University of Tasmania as Lead Agent.
Disclaimer:
You accept all risks and responsibility for losses, damages, costs and other consequences resulting directly or indirectly from using this site and any information or material available from it. While the Integrated Marine Observing System (IMOS) has taken reasonable steps to ensure that the information on this website and related publication is correct, it provides no warranty or guarantee that information provided by the authors is accurate, complete or up-to-date. IMOS does not accept any responsibility or liability for any actions taken as a result of, or in reliance on, information on its website or publication. Users should check with the originating authors to confirm the accuracy of the information before taking any action in reliance on that information.
If you believe any information on this website or in the related publication is inaccurate, out of date or misleading, please bring it to our attention by contacting the authors directly or emailing us at IMOS@imos.org.au
Images and Information:
All information on this website remains the property of those who authored it. All images on this website are licensed through Adobe Stock, Shutterstock, or have permission from the original owner.